Medicine details
| Image |  |
| Name | Polyforte |
| Dosage | Eye Drops |
| Generic Name | Neomycin Sulphate + Polymixin B Sulphate + Prednisolone |
| Classes |
Dermatological/Topical Agent Topical Antiinfective Agent Ophthalmic Preparation Antiinfective and Steroid Combination |
| Diseases |
Conjunctivitis Corneal Injury Ophthalmic Disease |
| Company | Aristopharma Limited |
Drug Package Details
| Strength | 350 mg + 127 mg + 500 mg/100 ml |
| Storage Condition | |
| Origin Country | Bangladesh |
| Commercial Pack | 1 |
| Price per pack | ৳ 149.48 |
| Cost per pack | ৳ 131.54 |
| Package unit | 5 ml drops |
| Price per unit | ৳ 149.48 |
| Cost per unit | ৳ 131.54 |
| Discount | 0 |
| Coupon | |
| Remarks |
Neomycin Sulphate + Polymixin B Sulphate + Prednisolone
Neomycin Sulphate + Polymixin B Sulphate + Prednisolone is a multiple dose anti-infective steroid combination in sterile suspension form for topical application. It is usually used to treat various inflammatory eye ophthalmic conditions.
Neomycin Sulphate + Polymixin B Sulphate + Prednisolone is indicated for the following conditions-
- Eye infections
- Conjunctivitis
- Uveitis
- Eye inflammation (due to chemical/mechanical injury)
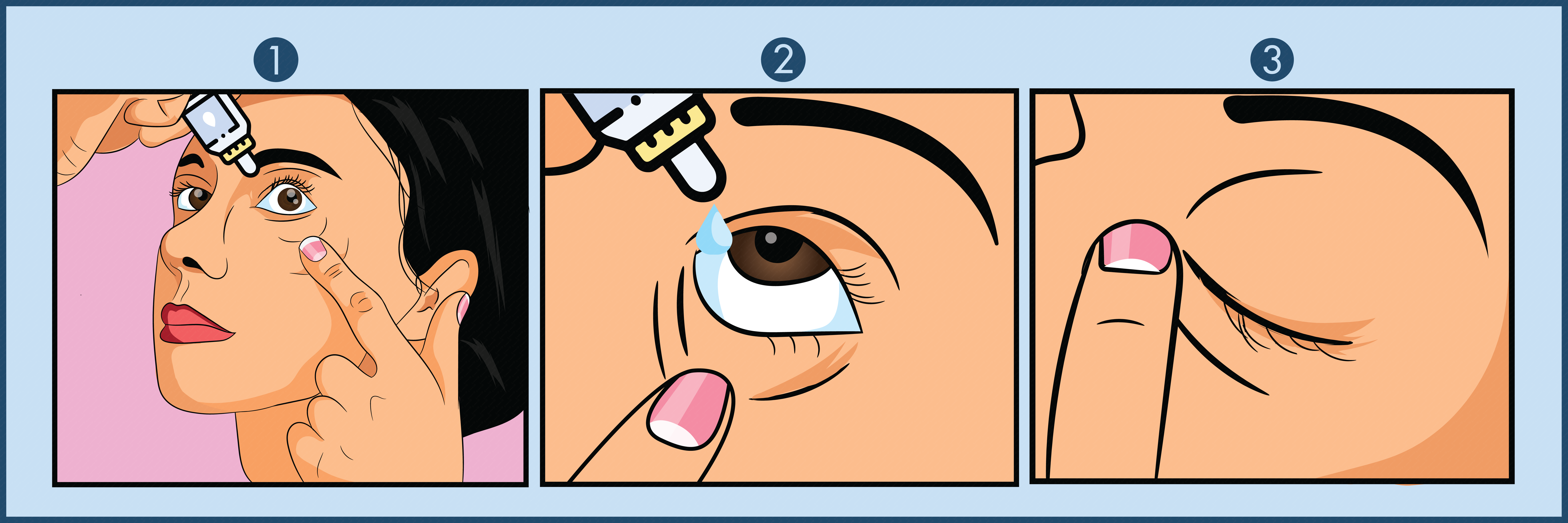
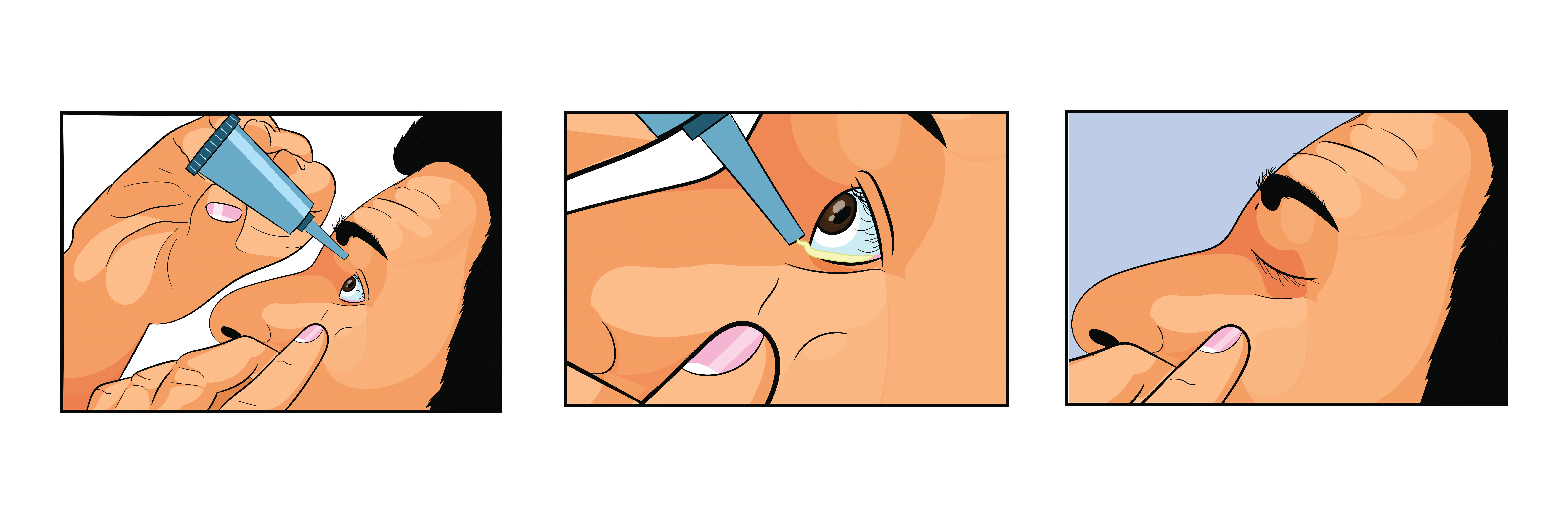
1–2 drops in the conjunctival sac (s). Drops may be used hourly in severe disease, tapering to discontinuation as the inflammation declines. Drops can be used up to four to six times per day for mild disease. In the beginning, no more than 20 mL should be prescribed, and the prescription should not be refilled without further evaluation.
| 1. Eye Drop | |
| 2. Eye ointment |
Adverse reactions to corticosteroid/anti-infective combination drugs have occurred, which can be attributed to the corticosteroid component, the anti-infective component, or the combination. Exact incidence figures are unavailable due to a lack of a denominator of treated patients.
- Ocular hypertension
- Glaucoma
- Optic nerve damage
- Cataract
- Delayed wound healing
- The initial prescription and renewal of the medication order beyond 20 mL of Neomycin Sulphate + Polymyxin B Sulphate + Prednisolone should be made by a physician only after a thorough examination of the patient using magnification, such as slit lamp bio-microscopy and, if necessary, fluorescein staining.
- If the patient's signs and symptoms do not improve after two days, he or she should be reevaluated.
- Because fungal infections of the cornea are more likely to develop concurrently with long-term corticosteroid use, fungal invasion should be suspected in any persistent corneal ulceration where a corticosteroid has been used or is being used.
- When necessary, fungal cultures should be obtained.
- IOP should be monitored if this product is used for more than 10 days.
- Prolonged use of topical anti-bacterial agents may give rise to overgrowth of non-susceptible organisms including fungi.
- If the inflammation or pain lasts more than 48 hours or worsens, the patient should be advised to stop taking the medication and consult a doctor.
Contraindication
Contraindicated in individuals with known or suspected hypersensitivity to any of the ingredients of this preparation and to other corticosteroids, such as-
None known.
Contraindicated in most viral diseases of the cornea and conjunctiva, including epithelial herpes simplex keratitis (dendritic keratitis), vaccinia, varicella, and also in mycobacterial infection of the eye and fungal diseases of ocular structures.
 Bangla
Bangla English
English